Natural Diamonds vs Synthetic Diamonds

Natural diamonds are a wonder of science, as they occur only when atoms of carbon are exposed to high pressures and temperatures about 100 miles (160 kilometers) below the earth’s surface in what geologists call the upper mantle. Diamond crystals reside in these extreme mantle conditions for millions of years. The crystals that grow under these conditions have a unique structure we associate with natural diamonds.
Natural diamond crystals sometimes incorporate solid small inclusions of diamonds or other minerals into their structure as they grow. While mineral inclusions are often considered a negative clarity feature in a polished diamond, they are of tremendous value to geoscientists. Natural diamonds provide a way for these mineral inclusions to be preserved and brought to the earth’s surface where they can be scientifically studied. Because certain inclusions contain radioactive elements that decay at a known rate, the minerals can also be used to calculate the age of diamond formation.
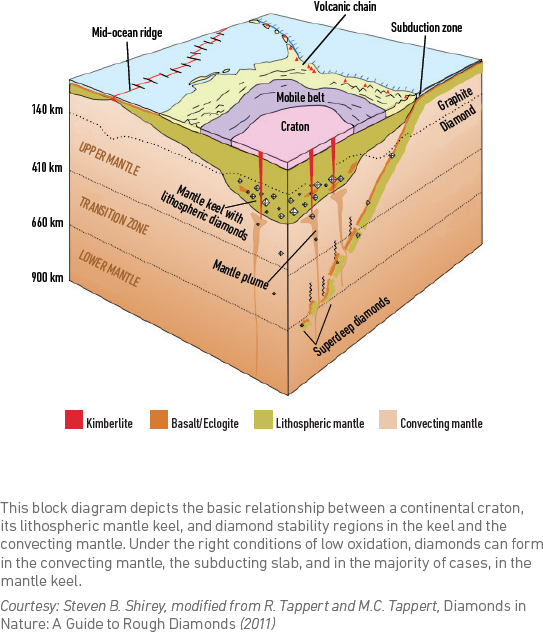
Following their extended time in residence in the mantle, some diamonds are brought to the earth’s surface by volcanic eruptions of kimberlite magma. Geologists believe that the diamonds are transported by the magma very quickly over the 100-mile distance (in just a week or more), so that the diamonds are not physically changed in the process and transformed to graphite. During this rapid upward journey, the diamond crystal can, however, break along cleavage directions, and undergo other changes that may affect, for example, its color.


Although their use for jewelry purposes is a somewhat recent occurrence, synthetic diamonds have been produced for industrial applications since the 1950s. Unlike natural diamonds, synthetic diamonds are grown in laboratories, over a very short period of time – likely just two or three weeks (or less). A longer growth period results in larger crystals; however, steady environmental conditions must be maintained to ensure the formation of high-quality crystals. Currently, the production of synthetic diamonds in melee sizes is what is most encountered. The drastically shorter growth period of synthetic diamonds results in features that are of diagnostic value in separating them from natural diamonds.
Two artificial growth methods are used to create synthetic diamonds: HPHT and CVD. HPHT (high pressure/high temperature) synthesis, developed in the 1950s, uses high temperatures and pressures, a molten metal flux and a diamond seed to initiate crystal formation. The result is a distinctive crystal shape which is a combination of octahedral and cube faces and a flat base. The HPHT process more closely mirrors the conditions of natural diamond formation than CVD.
The CVD (chemical vapor deposition) method, which was mostly developed during the past decade, produces diamonds through the use of low pressures and high temperatures in a vacuum chamber. A carboncontaining gas such as methane is introduced into the chamber, and gas molecules break down there into the constituent atoms. The carbon atoms “rain” down onto flat diamond seed plates, resulting in a square-shaped, tabular synthetic diamond crystal.


Various materials have been used as imitations or substitutes for diamonds since ancient times. These materials, often referred to as diamond simulants, may grow naturally in the earth or artificially in a lab. Years ago, the colorless varieties of some colored stones (i.e., quartz) were used to simulate diamond. Today, the most common simulants are cubic zirconia (zirconium oxide, or CZ), and to a lesser extent, synthetic moissanite (silicon carbide). Because simulants differ completely from diamond, they display diagnostic properties by which they can be recognized with standard gem testing techniques, observations under magnification and commercially available “diamond testers.”



